Why are biofilms a health threat ?
Up to 99% of bacteria exist in the form of biofilm.





Steps of biofilm formation
Biofilm formation is commonly considered to occur in five main stages :
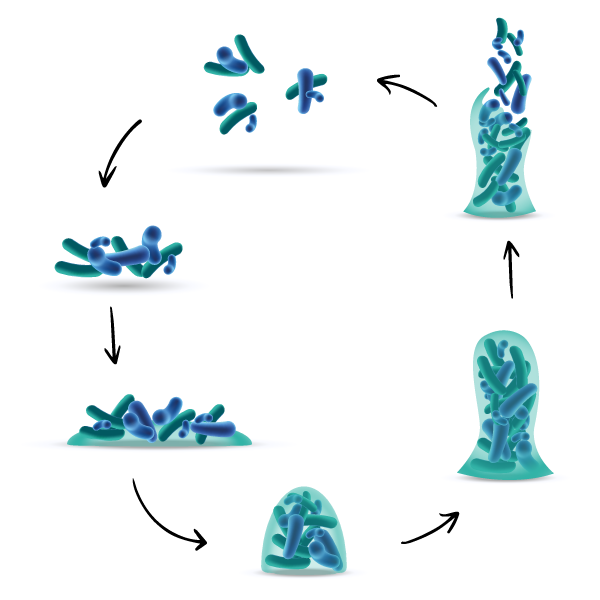
Biofilm formation is commonly considered to occur in five main stages :
- Adhesion : bacterial attachment to a surface
- Accumulation : microcolony formation
- Biofilm growth
- Biofilm maturation
- Dispersal of bacteria which may then colonize new areas.
Planktonic bacteria
1. Adhesion
Bacterial attachment to a surface
2. Accumulation
Microcolony formation
3. Biofilm growth
4. Biofilm maturation
5. Dispersal of bacteria
Which may then colonize new areas.
AMR ?
Antimicrobial resistance
Antimicrobial resistance remains even when calls are dispersed from biofilms.
In the hospital environment, biofilms have often been found to protect pathogens such as Staphylococcus aureus, Escherichia coli, Klebsiella or Candida spp., etc.¹
Biofilms are highly-structured polymicrobial communities embedded in a matrix adherent to surfaces. They consist mainly of polysaccharides, proteins, DNA and lipids.
1. RYDER, M. 2005
Properties of bacteria
(Incl. multi-drug restistant bacteria like MRSA, CRE, Pseudomonas Aeruginosa, Legionella, Viruses and Fungi).²
2. University of Southampton, Journal of Clinical Microbiology, Oct 2006

frequency
- Planktonic bacteria can begin to form a biofilm within minutes in contact with any interface.
- 99% of bacteria exist in the form of biofilm.³
- Biofilms are ubiquitous and develop frequently on Medical Devices (urinary and intravenous catheters, endoscopes, endoscope washers, dialyse circuits etc.).¹,⁴
- Tests can lead to “false-negative” results : germs hidden in biofilms are not collected.
- Marion, Freney, James, Bergeron, Renaud, Costerton : Using an efficient biofilm detaching agent: an essential step for the improvement of endoscope reprocessing protocols. Journal of Hospital Infection (2006) 64, 136 – 141
- Rendueles, O. & Ghigo, J.-M. Multi-species biofilms: how to avoid unfriendly neighbors. FEMS Microbiol. Rev. 36, 972‑989 (2012).
- Allison D G, Gilbert P, Lappin-Scott H M, Wilson M : Community Structure and Co-operation in Biofilms. Cambridge University Press, October 2000
- Donlan R M : Biofilm and Device-Associated Infections. Emerging Infectious Diseases, Vol. 7, No. 2, March–April 20011

emergent properties
- Biofilms have emergent properties (unpredictable from study of free, planktonic bacteria).⁵
- COOPERATION : Horizontal transfer of genes carrying antibiotics-resistance and virulence is favored inside biofilms.
- SURVIVAL : Biocides are mostly tested against free-floating (planktonic) bacteria, not against biofilms. Structural and functional properties of biofilm matrix enhance survival of exposure to antimicrobials.
- COMPLEX : Cells in biofilms have the ability to undergo differentiation. Continuously remodelled, every microbial species develops a specific matrix composition.
- Konopka A., 2009
biofilms enable bacteria to survive
in a wider range of condition
- Bacteria in biofilm up to 1000 times more tolerant of biocides (disinfectants).⁶
- Antibiotics-resistance is favored inside biofilms through cell-to-cell signaling mechanism (horizontal transfer of genes).
- Progressive accumulation leads to build-up of resistant biofilm over time. If the detergent action is not efficient against biofilm matrix, bacterial biofilm can resist high level disinfection.⁷
- Biofilms form a protective barrier around infectious microorganisms. Biofilms enhance survival of exposure to antimicrobials.⁸
- Rasmussen TB, Givskov M. Int J Med Microbiol 296(2–3):149–161 (2006).
- Berry et al, 2009 ; Gagnon et al, 2008 ; Norton et al, 2004 ; Williams et Braun-Howland, 2003
- Smith, K. & Hunter, I. S. Efficacy of common hospital biocides with biofilms of multi-drug resistant clinical isolates. J. Med. Microbiol. 57, 966‑973 (2008). / Vickery, K. et al. Presence of biofilm containing viable multiresistant organisms despite terminal cleaning on clinical surfaces in an intensive care unit. J. Hosp. Infect. 80, 52‑5 (2012). / Alfa, M. J. & Howie, R. Modeling microbial survival in buildup biofilm for complex medical devices. BMC Infect. Dis. 9, 56 (2009)
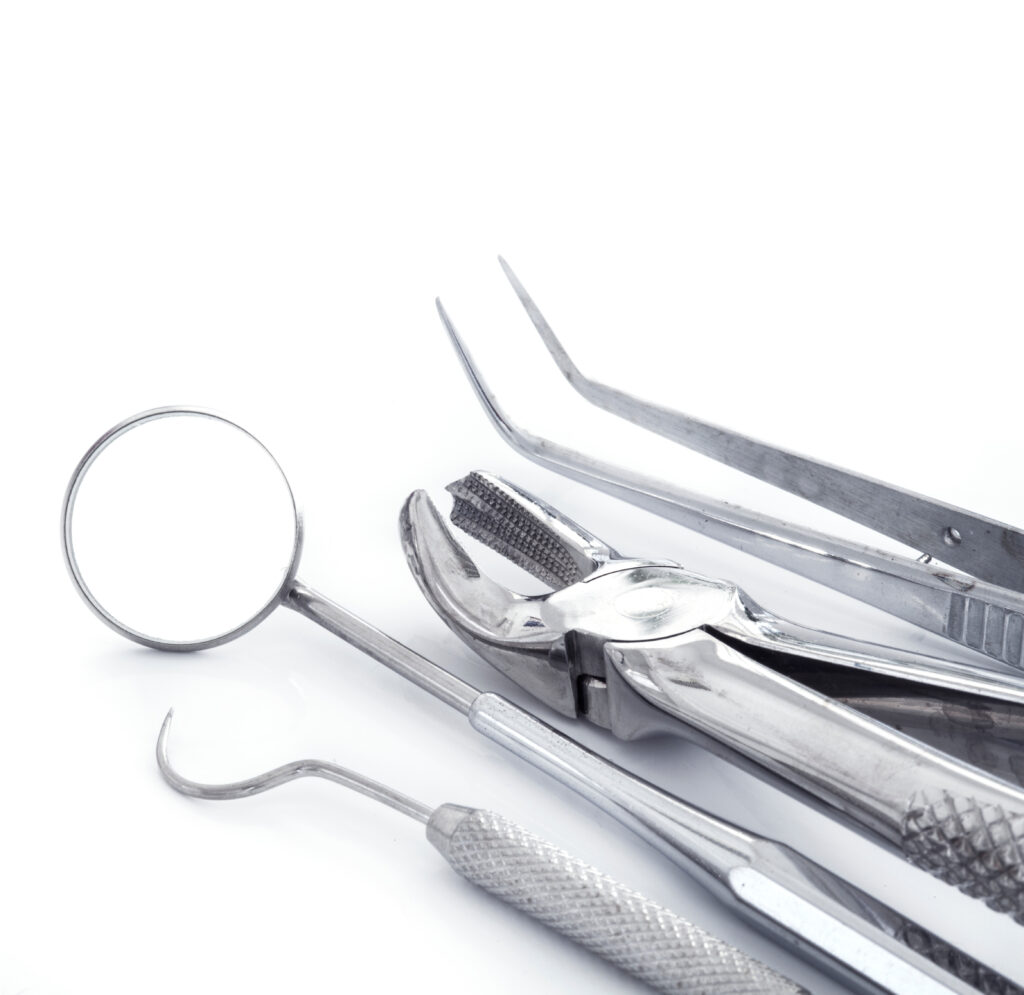
How to properly disinfect your instruments ?
Disinfection failures
/01
It is impossible to disinfect or even sterilize an inadequately cleaned instrument.10
/02
Biofilm matrix should be eliminated by the detergence process.
Microbes protected by biofilm will resist even high-level disinfection.
/03
Current Medical Device decontamination strategies assume that bacteria are free-floating (planktonic), whereas 99% of bacteria are protected by a biofilm.
Most biocides are tested against free (planktonic) bacteria, but not against biofilms.
/04
Inorganic and organic materials interfere with the effectiveness and antimicrobial activity of disinfectants and sterilization.9
9. CDC Guidelines for Disinfection and Sterilization in Healthcare Facilities, 2008
10. ESGE-ESGENA guidelines: Cleaning and disinfection in gastrointestinal endoscopy, 2008
In short, all disinfection processes, whether done manually or by washer-disinfector, should be done only after appropriate manual cleaning.
Applications

endoscopy
10 to 30% of patient-ready endoscopes are contaminated with microorganisms.

surgical instruments
Over 60% of correctly-processed surgical instruments exhibit residual protein soiling.

surfaces
Working in a safer & cleaner environment is a must. Take control.